A Phase Diagram Gives Information On
Phase diagram iron carbon phases diagrams steel equilibrium read single cast reading fec different gif boundaries composition Phase diagram chemistry features diagrams temperature point boundaries boundless liquid solid major water physics gas lines kelvin phases celsius changes Point phase eutectic rule lever diagrams calculations diagram muddiest material science materials line
How do you use a phase diagram? + Example
Major features of a phase diagram Phase temperature chemistry gas state changes diagrams curves shown heating diagram substance transition its temperatures pressure liquid solid graph labeled Phase diagrams
Phase diagrams
Carbonated drinks making ielts10.9: phase diagrams Muddiest point- phase diagrams i: eutectic calculations and lever ruleHow do you use a phase diagram? + example.
Phase diagramsPhase diagram with a triple point o of water analogy. Phase diagram change liquid matter line states graph melting curve solid chemistry freezing phases substance points pure diagrams boiling betweenFeatures of phase diagrams (m11q1) – uw-madison chemistry 103/104.

Phase substance pressure liquid melting critical state chem wisc unizin represented graphically temperatures physical sublimation vaporization
Phase changesPhase thermodynamic shown there Phase diagramsPhase diagram solid liquid rule pressure gibbs diagrams system binary read temperature chemistry phases substance h2o chem area which liquids.
Process diagram #9: the diagram gives information about the process ofPhase diagrams Phase diagrams diagram chemistry matter states general point which triple three figure transitions physical properties6.1.2 reading phase diagrams: single phases and boundaries.

Phase explanation
Phase diagramsPhase diagrams chemistry liquids diagram liquid solid gas supercritical phases region substance three general figure pressure solids fluid typical principles .
.


Phase Diagrams - YouTube
Phase Diagrams

Phase diagram with a triple point O of water analogy. | Download

6.1.2 Reading Phase Diagrams: Single Phases and Boundaries

Process diagram #9: The diagram gives information about the process of
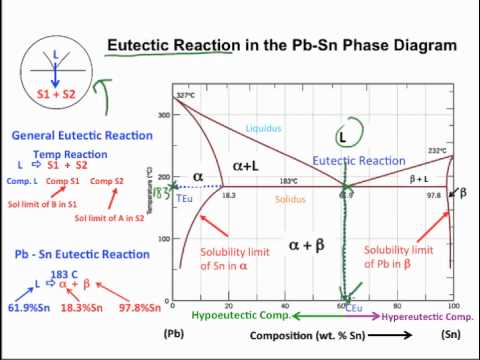
Muddiest Point- Phase Diagrams I: Eutectic Calculations and Lever Rule

Phase Diagrams | Chemistry for Majors

How do you use a phase diagram? + Example

10.9: Phase Diagrams - Chemwiki